Uranium(III) iodide
Identifiers
CAS Number
3D model (JSmol )
ChemSpider
ECHA InfoCard
100.033.992
EC Number
PubChem CID
CompTox Dashboard (EPA )
InChI
InChI=1S/3HI.U/h3*1H;/q;;;+3/p-3Key: CDQDFXDBVYMPJX-UHFFFAOYSA-K
SMILES
Properties
Chemical formula
UI3
Molar mass
618.74232 g/mol
Appearance
black solid
Density
6.78 g/cm
Melting point
766 °C (1,411 °F; 1,039 K)
Structure
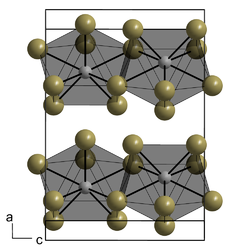
Crystal structure
orthorhombic
Space group
Ccmm, No. 63
Lattice constant
a = 432.8 pm, b = 1401.1 pm, c = 1000.5 pm
Formula units (Z )
4
Hazards
GHS labelling
Pictograms
Signal word
Danger
Hazard statements
H300 , H330 , H373 , H411
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
Infobox references
Chemical compound
Uranium triiodide is an inorganic compound with the chemical formula UI3 . It is a black solid that is soluble in water.
Production
Uranium triiodide can be obtained from the direct reaction of its constituent elements:
2 U + 3 I2 → 2 UI3 When the reaction is conducted in tetrahydrofuran (THF), the product is the blue complex UI3 (THF)4 .
Properties
It crystallizes in the orthorhombic crystal system (plutonium tribromide -type) in the space group Ccmm with the lattice parameters a = 432.8 pm, b = 1401.1 pm, and c = 1000.5 pm and four formula units per unit cell .
Uranium triiodide can be used as a Lewis acid catalyst for various Diels-Alder reactions carried out under mild conditions.
References
^ Levy, J. H.; Taylor, J. C.; Wilson, P. W. (1975-03-01). "The structure of uranium(III) triiodide by neutron diffraction" . Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry . 31 (3): 880–882. Bibcode :1975AcCrB..31..880L . doi :10.1107/S0567740875003986 . ISSN 0567-7408 .
Lehrbuch der Anorganischen Chemie , 102. Auflage, de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , S. 1969.
Handbuch der präparativen anorganischen Chemie. 2 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1978. ISBN 978-3-432-87813-3 David L. Clark; Alfred P. Sattelberger (1996). "Lewis Base Adducts of Uranium Triiodide and Tris[Bis(Trimethylsilyl)Amido]Uranium". Inorganic Syntheses . Inorganic Synththesis. Vol. 31. pp. 307–315. doi :10.1002/9780470132623.ch55 . ISBN 978-0-470-13262-3
Collin, Jacqueline; Maria, Leonor; Santos, Isabel (Oct 2000). "Uranium iodides as catalysts for Diels–Alder reactions" . Journal of Molecular Catalysis A: Chemical . 160 (2): 263–267. doi :10.1016/S1381-1169(00)00257-0 .
Salts and covalent derivatives of the iodide ion
Categories :
Uranium(III) iodide
Add topic
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑