 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Manganese(II) iodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.274 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | MnI2 |
| Molar mass | 308.747 g/mol |
| Appearance | pink crystalline |
| Density | 5.01 g/cm |
| Melting point | 701 °C (1,294 °F; 974 K) (anhydrous) 80 °C (tetrahydrate) |
| Boiling point | 1,033 °C (1,891 °F; 1,306 K) |
| Solubility in water | soluble |
| Magnetic susceptibility (χ) | +14,400·10 cm/mol |
| Structure | |
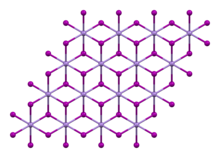
| Crystal structure | Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 |
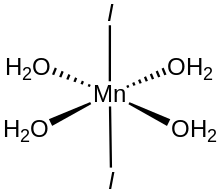
| Coordination geometry | octahedral |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H360 |
| Precautionary statements | P201, P202, P281, P308+P313, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | non-flammable |
| Related compounds | |
| Other anions | Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
| Other cations | Iron(II) iodide Cobalt(II) iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Manganese(II) iodide is the chemical compound composed of manganese and iodide with the formula MnI2(H2O)n. The tetrahydrate is a pink solid while the anhydrous derivative is beige. Both forms feature octahedral Mn centers. Unlike MnCl2(H2O)4 and MnBr2(H2O)4 which are cis, MnI2(H2O)4 is trans.
Preparation
Anhydrous MnI2 is prepared from the elements:
- Mn + I2 → MnI2
The tetrahydrate can be prepared by treating manganese(II) carbonate with hydriodic acid. The anhydrous form can be produced from it by dehydration in a vacuum.
Properties
Samples turn brown in air under the influence of light as a result of the oxidation of the iodide ion to iodine. It has a trigonal crystal structure of the cadmium iodide type (polytype 2H) with the space group P3m1 (space group no. 164). It dissolves in water and decomposes. The tetrahydrate has a monoclinic crystal structure with the space group P21/c (No. 14).
Applications
It is often used in the lighting industry.
References
- "223646 Manganese(II) iodide 98%". Sigma-Aldrich. Retrieved 2011-08-05.
- ^ Hosseiny, Afshin; MacKie, Anthony G.; McAuliffe, Charles A.; Minten, Karl (1981). "The Coordination Chemistry of Manganese". Inorganica Chimica Acta. 49: 99–105. doi:10.1016/S0020-1693(00)90464-X.
- ^ Moore, J. E.; Abola, J. E.; Butera, R. A. (1985-09-15). "Structure of manganese(II) iodide tetrahydrate, MnI2.4H2O". Acta Crystallographica Section C: Crystal Structure Communications. 41 (9): 1284–1286. doi:10.1107/S0108270185007466. ISSN 0108-2701.
- Friour, G.; Cahiez, G.; Normant, J. F. (1984). "Organomanganous Reagents; IX. Preparation of Various Halogenated, Alkoxylated, Aryloxylated, and Arylsulfenylated Ketones from Correspondingly Functionalized Carboxylic Acid Chlorides or Anhydrides". Synthesis. 1984: 37–40. doi:10.1055/s-1984-30724. S2CID 94812612.
- ^ Ans, Jan d'; Ans, Jan d' (1998). Elemente, anorganische Verbindungen und Materialien. Taschenbuch für Chemiker und Physiker / D'Ans (4., neubearb. u. rev. Aufl ed.). Ort nicht ermittelbar: Verlag nicht ermittelbar. ISBN 978-3-540-60035-0.
- Riedel, Erwin; Alsfasser, Ralf, eds. (2007). Moderne anorganische Chemie: mit CD-ROM (3. Aufl ed.). Berlin: de Gruyter. ISBN 978-3-11-019060-1.
- Cable, J. W.; Wilkinson, M. K.; Wollan, E. O.; Koehler, W. C. (1962). "Neutron Diffraction Investigation of the Magnetic Order in MnI2". Phys. Rev. 125 (6): 1860–1864. doi:10.1103/PhysRev.125.1860.
- Cepanec, Ivica (2004). Synthesis of Biaryls. Elsevier. p. 104. ISBN 0-08-044412-1. Retrieved 2008-06-18.
| Manganese compounds | |
|---|---|
| Manganese(−I) | |
| Manganese(0) | |
| Manganese(I) | |
| Manganese(II) | |
| Manganese(II,III) | |
| Manganese(II,IV) | |
| Manganese(III) | |
| Manganese(IV) | |
| Manganese(V) | |
| Manganese(VI) | |
| Manganese(VII) | |
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||