 | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | RbI3 |
| Appearance | black crystals |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Rubidium triiodide is an inorganic compound with the chemical formula RbI3. It is composed of Rb and I
3.
Preparation
Rubidium triiodide can be obtained by heating rubidium iodide and iodine in aqueous solution:
- RbI + I2 → RbI3
Properties
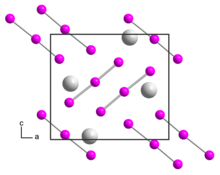
Rubidium triiodide is an orthorhombic black crystal, isomorphic to caesium triiodide, with space group Pnma, unit cell parameters a = 1090.8 pm, b = 665.5 pm, c = 971.1 pm. Upon heating to 270 °C, the rubidium triiodide decomposes into rubidium iodide and elemental iodine. It is soluble in ethanol and decomposes in ether.
Reactions
It has long been believed that rubidium triiodide reacts further with iodine to form RbI7 and RbI9, but this has been refuted by recent studies.
References
- ^ Wells, H. L.; Wheeler, H. L.; Penfield, S. L. (1892). "Über Trihalogenverbindungen des Rubidiums und Kaliums. nebst ihrer Krystallographie". Zeitschrift für anorganische Chemie. 1 (1): 442–455. doi:10.1002/zaac.18920010140.
- ^ Tebbe, K. F.; Georgy, U. (1986-12-15). "Die Kristallstruckturen von Rubidiumtriiodid und Thalliumtriiodid". Acta Crystallographica Section C Crystal Structure Communications. 42 (12). International Union of Crystallography (IUCr): 1675–1678. doi:10.1107/s0108270186090972. ISSN 0108-2701.
- Abegg, R. (1906-09-21). "Über die festen Polyjodide der Alkalien, ihre Stabilität und Existenzbedingungen bei 25°". Zeitschrift für anorganische Chemie. 50 (1). Wiley: 403–438. doi:10.1002/zaac.19060500136. ISSN 0863-1778.
- Briggs, T. R.; Patterson, E. S. (1932-10-01). "The Polyiodides of Rubidium. I". The Journal of Physical Chemistry. 36 (10). American Chemical Society (ACS): 2621–2624. doi:10.1021/j150340a011. ISSN 0092-7325.
| Rubidium compounds | |
|---|---|
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||