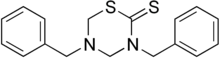 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.907 |
| Chemical and physical data | |
| Formula | C17H18N2S2 |
| Molar mass | 314.46 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Sulbentine (or dibenzthione) is an antifungal.
References
- Hänel H, Raether W, Dittmar W (1988). "Evaluation of fungicidal action in vitro and in a skin model considering the influence of penetration kinetics of various standard antimycotics". Annals of the New York Academy of Sciences. 544 (1): 329–37. Bibcode:1988NYASA.544..329H. doi:10.1111/j.1749-6632.1988.tb40417.x. PMID 3214073. S2CID 13183316.
This dermatologic drug article is a stub. You can help Misplaced Pages by expanding it. |