 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
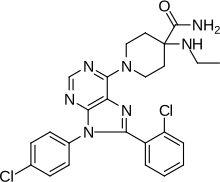
| Formula | C25H25Cl2N7O |
| Molar mass | 510.42 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Otenabant (CP-945,598) is a drug that acts as a potent and highly selective CB1 antagonist. It was developed by Pfizer for the treatment of obesity, but development for this application has been discontinued following the problems seen during clinical use of the similar drug rimonabant.
See also
References
- Kim MA, Yun H, Kwak H, Kim J, Lee J (2008). "Design, chemical synthesis, and biological evaluation of novel triazolyl analogues of taranabant (MK-0364), a cannabinoid-1 receptor inverse agonist". Tetrahedron. 64 (48): 10802–10809. doi:10.1016/j.tet.2008.09.057.
- Woods SC (November 2007). "The endocannabinoid system: novel pathway for cardiometabolic Risk-factor reduction". Journal of the American Academy of Physician Assistants. Suppl Endocannabinoid (11): 7–10. doi:10.1097/01720610-200711000-00005. PMID 18047036. S2CID 25472128.
- "Pfizer Pharmaceutical News and Media - Pfizer: One of the world's premier biopharmaceutical companies". www.pfizer.com.
| Cannabinoids | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytocannabinoids (comparison) |
| ||||||||||||||||||||||||||||||||||||||||
| Endocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||
| Synthetic cannabinoid receptor agonists / neocannabinoids |
| ||||||||||||||||||||||||||||||||||||||||
| Allosteric CBRTooltip Cannabinoid receptor ligands | |||||||||||||||||||||||||||||||||||||||||
| Endocannabinoid enhancers (inactivation inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||
| Anticannabinoids (antagonists/inverse agonists/antibodies) |
| ||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Cannabinoid receptor modulators | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
This cannabinoid related article is a stub. You can help Misplaced Pages by expanding it. |