 | |
 | |
| Names | |
|---|---|
| IUPAC name tetrabromostannate | |
| Other names tin tetrabromide, stannic bromide, bromostannic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.258 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | SnBr4 |
| Molar mass | 438.33 g/mol |
| Appearance | colourless |
| Density | 3.340 g/cm (at 35 °C) |
| Melting point | 31 °C (88 °F; 304 K) |
| Boiling point | 205 °C (401 °F; 478 K) |
| Solubility in water | soluble |
| Magnetic susceptibility (χ) | −149.0·10 cm/mol |
| Related compounds | |
| Other anions | Tin(IV) fluoride Tin(IV) chloride Tin(IV) iodide |
| Other cations | Carbon tetrabromide Silicon tetrabromide Germanium tetrabromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tin(IV) bromide is the chemical compound SnBr4. It is a colourless low melting solid.
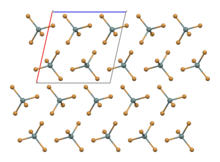
Structure
SnBr4 occurs in form of crystals. The compound crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry, with mean Sn-Br bond lengths of 242.3 pm.
Preparation
SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP):
- Sn + 2Br
2 → SnBr
4
Dissolution in solvents
In aqueous solution SnBr4 dissolves to give a series of octahedral (six-ligated) bromo-aquo complexes. These include SnBr4(H2O)2 and cis- and trans-[SnBr2(H2O)4].
Reactions
SnBr4 forms 1:1 and 1:2 complexes with ligands, e.g. with trimethylphosphine the following can be produced, SnBr4.P(CH3)3 and SnBr4.2P(CH3)3.
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Brand, P.; Sackmann, H. (1963). "Die Kristallstruktur von SnBr4" [The crystal structure of SnBr4]. Acta Crystallographica (in German). 16 (6): 446–451. Bibcode:1963AcCry..16..446B. doi:10.1107/S0365110X63001250.
- Reuter, H.; Pawlak, R. (2001). "Zinnhalogenverbindungen. II. Die Molekül- und Kristallstrukturen von Zinn(IV)-bromid und -iodid" [Tin halogen compounds. II. The Molecular and Crystal Structures of Tin(IV) Bromide and Tin(IV) Iodide]. Zeitschrift für Kristallographie – Crystalline Materials (in German). 216 (1–2001): 34–38. Bibcode:2001ZK....216...34R. doi:10.1524/zkri.216.1.34.18992. S2CID 94609783.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic Chemistry. Academic Press, Elsevier. ISBN 978-0-12-352651-9. OCLC 1024925228.
- Taylor, M. J.; Coddington, J. M. (1992). "The constitution of aqueous tin(IV) chloride and bromide solutions and solvent extracts studied by Sn NMR and vibrational spectroscopy". Polyhedron. 11 (12): 1531–1544. doi:10.1016/S0277-5387(00)83148-4.
- Frieson, D. K.; Ozin, G. A. (1973). "Preparation, Infrared and Raman Spectra, and Stereochemistries of Pentacoordinate Trimethylphosphine Complexes, MX4•P(CH3)3 and MX4•P(CD3)3 where M = Ge or Sn and X = Cl or Br". Canadian Journal of Chemistry. 51 (16): 2697–2709. doi:10.1139/v73-406.
| Tin compounds | |
|---|---|
| Sn(II) | |
| Sn(IV) | |
| Bromine compounds | |
|---|---|
| Br(−I) | |
| Br(−I,I) | |
| Br(I) | |
| Br(II) | |
| Br(I,V) | |
| Br(III) | |
| Br(IV) | |
| Br(V) | |
| Br(VII) | |