 | |
| Clinical data | |
|---|---|
| Other names | SC-23133; 3-(17β-Hydroxy-6β,7β-methylene-3-oxo-4-androsten-17α-yl)propionic acid γ-lactone |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H30O3 |
| Molar mass | 354.490 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
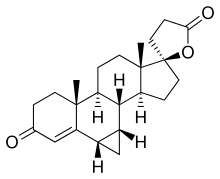
Prorenone (developmental code name SC-23133) is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of prorenoic acid (prorenoate), and prorenoate potassium (SC-23992), the potassium salt of prorenoic acid, also exists. Prorenoate potassium is about 8 times more potent than spironolactone as an antimineralocorticoid in animals, and it may act as a prodrug to prorenone. In addition to the mineralocorticoid receptor, prorenone also binds to the glucocorticoid, androgen, and progesterone receptors. The antiandrogenic potency of prorenone in vivo in animals is close to that of spironolactone. Similarly to spironolactone, prorenone is also a potent inhibitor of aldosterone biosynthesis.
Chemistry
Synthesis
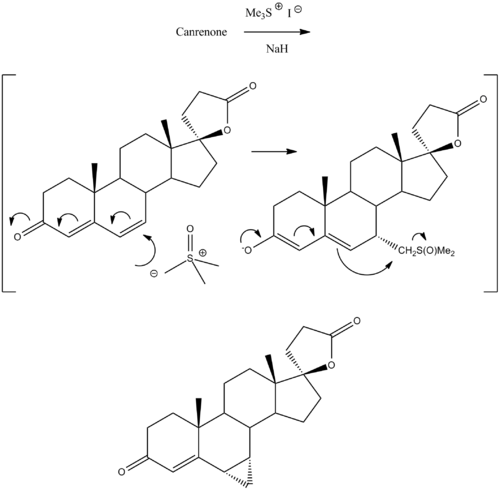
Prorenone can be synthesized via a Johnson–Corey–Chaykovsky reaction by reaction of canrenone with trimethylsulfoxonium iodide and sodium hydride.
See also
References
- ^ Claire M, Rafestin-Oblin ME, Michaud A, Roth-Meyer C, Corvol P (April 1979). "Mechanism of action of a new antialdosterone compound, prorenone". Endocrinology. 104 (4): 1194–1200. doi:10.1210/endo-104-4-1194. PMID 436757.
- Szasz G, Budvari-Barany Z (19 December 1990). Pharmaceutical Chemistry of Antihypertensive Agents. CRC Press. pp. 87–. ISBN 978-0-8493-4724-5.
- ^ Kamata S, Matsui T, Haga N, Nakamura M, Odaguchi K, Itoh T, et al. (September 1987). "Aldosterone antagonists. 2. Synthesis and biological activities of 11,12-dehydropregnane derivatives". Journal of Medicinal Chemistry. 30 (9): 1647–1658. doi:10.1021/jm00392a022. PMID 3040999.
- Netchitailo P, Delarue C, Perroteau I, Leboulenger F, Capron MH, Vaudry H (January 1985). "Relative inhibitory potency of five mineralocorticoid antagonists on aldosterone biosynthesis in vitro". Biochemical Pharmacology. 34 (2): 189–194. doi:10.1016/0006-2952(85)90123-6. PMID 2981534.
- US 3845041, Chinn L, "7-Halomethyl-17-hydroxy-3-oxo-17alpha-pregn-4-ene-21-carboxylic acid gamma-lactones", issued 19 October 1974, assigned to GD Searle LLC.
| Mineralocorticoid receptor modulators | |||||
|---|---|---|---|---|---|
| MRTooltip Mineralocorticoid receptor |
| ||||
| Progesterone receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| PRTooltip Progesterone receptor |
| ||||||
| mPRTooltip Membrane progesterone receptor (PAQRTooltip Progestin and adipoQ receptor) |
| ||||||
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |