
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides.
Anions of this group, with the structure R3P=N, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions.
Synthesis
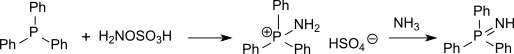
Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt.
Reactions and applications
The functional group will readily hydrolyse to give a phosphine oxide and an amine
- R3P=NR' + H2O → R3P=O + R'NH2
Phosphinimide ligands of the general formula NPR3 form transition metal phosphinimide complexeses. Some of these complexes are potential catalysts for the synthesis of polyethylene.
See also
References
- ^ Dehnicke, Kurt; Krieger, Matthias; Massa, Werner (February 1999). "Phosphoraneiminato complexes of transition metals". Coordination Chemistry Reviews. 182 (1): 19–65. doi:10.1016/S0010-8545(98)00191-X.
- Appel, Rolf; Büchner, Werner; Guth, Egbert (26 November 1958). "Zur Kenntnis des Imins, I. Über Phosphinimine und Sulfimine". Justus Liebigs Annalen der Chemie. 618 (1): 53–58. doi:10.1002/jlac.19586180107.
| Functional groups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons (only C and H) | |||||||||||||||
| Only carbon, hydrogen, and oxygen (only C, H and O) |
| ||||||||||||||
| Only one element, not being carbon, hydrogen, or oxygen (one element, not C, H or O) |
| ||||||||||||||
| Other | |||||||||||||||