 | |
| Names | |
|---|---|
| Other names 2-bromomesitylene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.008.552 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H11Br |
| Molar mass | 199.091 g·mol |
| Appearance | colorless liquid |
| Density | 1.3220 g/cm |
| Melting point | −1 °C (30 °F; 272 K) |
| Boiling point | 225 °C (437 °F; 498 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
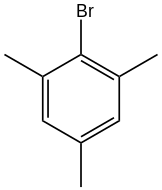
Mesityl bromide is an organic compound with the formula (CH3)3C6H2Br. It is a derivative of mesitylene (1,3,5-trimethylbenzene) with one ring H replaced by Br. The compound is a colorless oil. It is a standard electron-rich aryl halide substrate for cross coupling reactions. With magnesium it reacts to give the Grignard reagent, which is used in the preparation of tetramesityldiiron.
It is prepared by the direct reaction of bromine with mesitylene:
- (CH3)3C6H3 + Br2 → (CH3)3C6H2Br + HBr
References
- "2-Bromomesitylene". pubchem.ncbi.nlm.nih.gov.
- Farina, Vittorio; Krishnamurthy, Venkat; Scott, William J. (1997). "The Stille Reaction". Organic Reactions. pp. 1–652. doi:10.1002/0471264180.or050.01. ISBN 0471264180.
- Lee Irvin Smith (1931). "Isoodurene". Org. Synth. 11: 66. doi:10.15227/orgsyn.011.0066.
- Lee Irvin Smith (1931). "Bromomesitylene". Org. Synth. 11: 24. doi:10.15227/orgsyn.011.0024.