 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 5 hours |
| Excretion | Renal (98%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
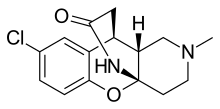
| Formula | C15H17ClN2O2 |
| Molar mass | 292.76 g·mol |
| 3D model (JSmol) | |
SMILES
| |
Lortalamine (LM-1404) is an antidepressant which was synthesized in the early 1980s. It acts as a potent and highly selective norepinephrine reuptake inhibitor. Lortalamine was under development for clinical use but was shelved, likely due to the finding that it produced ocular toxicity in animals. It has been used to label the norepinephrine transporter in positron emission tomography studies.
See also
References
- David J. Triggle (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9.
- Belleville M, Grand M, Briet P (1981). "Plasma levels, elimination and metabolic fate of 4a-amino-8-chloro-2-methyl-1,2,3,4,4a,10a-hexahydro-10H-benzopyrano[3,2-c]pyridin-10-ylacetic acid lactam, a new antidepressive agent, in rats and dogs". Drug Metabolism and Disposition. 9 (3): 233–9. PMID 6113932.
- Depin JC, Betbeder-Matibet A, Bonhomme Y, Muller AJ, Berthelon JJ (1985). "Pharmacology of lortalamine, a new potent non-tricyclic antidepressant". Arzneimittel-Forschung. 35 (11): 1655–62. PMID 4091869.
- ^ Lin KS, Ding YS (August 2005). "Synthesis and C-11 labeling of three potent norepinephrine transporter selective ligands ((R)-nisoxetine, lortalamine, and oxaprotiline) for comparative PET studies in baboons". Bioorganic & Medicinal Chemistry. 13 (15): 4658–66. doi:10.1016/j.bmc.2005.04.062. PMID 15914010.
- Elsom LF, Biggs SR, Chasseaud LF, Hawkins DR, Pulsford J, Darragh A (1985). "Metabolism of the anti-depressant lortalamine". European Journal of Drug Metabolism and Pharmacokinetics. 10 (3): 209–15. doi:10.1007/bf03189744. PMID 4085522. S2CID 2196891.
- Mally C, Thiebault JJ (1990). "Ocular toxicity in beagle dogs with lortalamine, a non tricyclic antidepressant compound". Drug and Chemical Toxicology. 13 (4): 309–23. doi:10.3109/01480549009032289. PMID 2279460.
- Ding YS, Lin KS, Logan J, Benveniste H, Carter P (July 2005). "Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S,S) and (R,R) enantiomers of reboxetine analogs ([11C]methylreboxetine, 3-Cl-[11C]methylreboxetine and [18F]fluororeboxetine), (R)-[11C]nisoxetine, [11C]oxaprotiline and [11C]lortalamine". Journal of Neurochemistry. 94 (2): 337–51. doi:10.1111/j.1471-4159.2005.03202.x. PMID 15998285. S2CID 25110597.
- Ding YS, Lin KS, Logan J (2006). "PET imaging of norepinephrine transporters". Current Pharmaceutical Design. 12 (30): 3831–45. doi:10.2174/138161206778559687. PMID 17073682.
| Monoamine reuptake inhibitors | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DATTooltip Dopamine transporter (DRIsTooltip Dopamine reuptake inhibitors) |
| ||||||||||||||
| NETTooltip Norepinephrine transporter (NRIsTooltip Norepinephrine reuptake inhibitors) |
| ||||||||||||||
| SERTTooltip Serotonin transporter (SRIsTooltip Serotonin reuptake inhibitors) |
| ||||||||||||||
| VMATsTooltip Vesicular monoamine transporters | |||||||||||||||
| Others |
| ||||||||||||||
| See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||