 | |
| Names | |
|---|---|
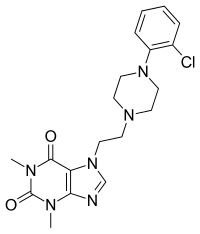
| Preferred IUPAC name 7-{2-ethyl}-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione | |
| Other names 7-ethyl]-1,3-dimethylxanthine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.211.655 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H23ClN6O2 |
| Molar mass | 402.88 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
KMUP-1 is a xanthine derivative with phosphodiesterase inhibitor activity.
References
- Liou, Shu-Fen; Hsu, Jong-Hau; Lin, I-Ling; Ho, Mei-Ling; Hsu, Pei-Chuan; Chen, Li-Wen; Chen, Ing-Jun; Yeh, Jwu-Lai (2013). "KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: roles of MAPKs, Akt, NF-κB and calcium/calcineurin/NFATc1 pathways". PLOS ONE. 8 (7): e69468. Bibcode:2013PLoSO...869468L. doi:10.1371/journal.pone.0069468. PMC 3723916. PMID 23936022.