 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.659 |
| Chemical and physical data | |
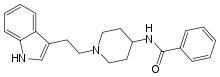
| Formula | C22H25N3O |
| Molar mass | 347.462 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Indoramin (trade names Baratol and Doralese) is a piperidine antiadrenergic agent.
It is an alpha-1 selective adrenoceptor antagonist with direct myocardial depression action; therefore, it results in no reflex tachycardia. It is also used in benign prostatic hyperplasia (BPH).
It is commonly synthesized from tryptophol.
Dosage
Indoramin is commonly prescribed as 20 mg tablets when used in BPH.
Side Effects
Drowsiness, dizziness, dry mouth, nasal congestion, headache, fatigue, weight gain, hypotension, postural hypotension, depression, problems with ejaculation, diarrhoea, nausea, increased need to pass urine, and palpitations.
Synthesis
Tryptamine and serotonin are naturally occurring indole ethylamino compounds with pronounced pharmacological activities. They have served as the inspiration for synthesis of numerous analogues.
One such study involved alkylation of 4-benzamidopyridine (2) with a bromoethyy compound (1) derived from tryptophol, to give a quaternary pyridinium salt (3); this intermediate was in turn hydrogenated with a Raney nickel catalyst to give indoramine.
Product withdrawal
On May 31, 2013, the French National Agency for the Safety of Medicines and Health Products (ANSM) concluded that the benefit/risk ratio of this product was unfavorable and withdrew Vidora's marketing authorization and recalled its batches from the market on June 3, 2013.
References
- Pierce V, Shepperson NB, Todd MH, Waterfall JF (February 1986). "Investigation into the cardioregulatory properties of the alpha 1-adrenoceptor blocker indoramin". British Journal of Pharmacology. 87 (2): 433–441. doi:10.1111/j.1476-5381.1986.tb10834.x. PMC 1916533. PMID 3955309.
- "Indoramin 20mg tablets". Medicines.org.uk. April 20, 2011. Archived from the original on July 25, 2022. Retrieved September 30, 2012.
- Ullman's encyclopedia of Industrial Chemistry, Sixth Edition, 2002.
- "Indoramin hydrochloride". National Health Service (UK). Retrieved September 30, 2012.
- "Indoramin 20mg tablets". Medicines.org.uk. Retrieved February 7, 2018.
- ZA 6803204, Archibald JL, Jackson JO ; eidem, U.S. patent 3,527,761 (1969, 1970 both to Wyeth).
- Archibald JL, Alps BJ, Cavalla JF, Jackson JL (November 1971). "Synthesis and hypotensive activity of benzamidopiperidylethylindoles". Journal of Medicinal Chemistry. 14 (11): 1054–1059. doi:10.1021/jm00293a009. PMID 5115203.
- "Actualités". ANSM (in French). Retrieved 2023-04-17.
| Sympatholytic (and closely related) antihypertensives (C02) | |||||
|---|---|---|---|---|---|
| Sympatholytics (antagonize α-adrenergic vasoconstriction) | |||||
| Other antagonists |
| ||||
| |||||
| Adrenergic receptor modulators | |||||
|---|---|---|---|---|---|
| α1 |
| ||||
| α2 |
| ||||
| β |
| ||||
This antihypertensive-related article is a stub. You can help Misplaced Pages by expanding it. |
