 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name 4-(4-hydroxyphenyl)iminocyclohexa-2,5-dien-1-one | |
| Other names Benzenoneindophenol, phenolindophenol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.194 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H9NO2 |
| Molar mass | 199.209 g·mol |
| Appearance | Reddish-blue powder |
| Melting point | above 300 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
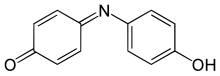
Indophenol is an organic compound with the formula OC6H4NC6H4OH. It is deep blue dye that is the product of the Berthelot's reaction, a common test for ammonia. The indophenol group, with various substituents in place of OH and various ring substitutions, is found in many dyes used in hair coloring and textiles.
Indophenol is used in hair dyes, lubricants, redox materials, liquid crystal displays, fuel cells and chemical-mechanical polishing. It is an environmental pollutant and is toxic to fish.
Berthelot test
In the Berthelot test (1859), a sample suspected of containing ammonia is treated with sodium hypochlorite and phenol. The formation of indophenol is used to determine ammonia and paracetamol by spectrophotometry. Other phenols can be used. Dichlorophenol-indophenol (DCPIP), a form of indophenol, is often used to determine the presence of vitamin C (ascorbic acid).
Related compounds
Indophenol blue is a different compound with systematic name N-(p-dimethylaminophenyl)-1,4-naphthoquinoneimine.

References
- ^ Sabnis, R. W. (2007). Handbook of Acid-Base Indicators. CRC Press. p. 196. ISBN 978-0-8493-8219-2.
- Patton, Charles J.; Crouch, S. R. (1977). "Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia". Analytical Chemistry. 49 (3): 464–469. doi:10.1021/ac50011a034.
- Horst Berneth (2002). "Azine Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_213.pub2. ISBN 978-3527306732.
- "Indophenol I5763". 500-85-6. Retrieved 2020-06-11.
- Tsuboi, T.; Hirano, Y.; Shibata, Y.; Motomizu, S. (2002). "Sensitivity improvement of ammonia determination based on flow-injection indophenol spectrophotometry with manganese(II) ion as a catalyst and analysis of exhaust gas of thermal power plant". Analytical Sciences. 18 (10): 1141–4. PMID 12400662.
- Hughes, David Emlyn (1983). "Titrimetric Determination of Ascorbic Acid with 2,6-Dichlorophenol Indophenol in Commercial Liquid Diets". Journal of Pharmaceutical Sciences. 72 (2): 126–129. doi:10.1002/jps.2600720208.
- Graham, S. O. (1963). "Indophenol Blue as a Chromogenic Agent for Identification of Halogenated Aromatic Hydrocarbons". Science. 139 (3557): 835–836. Bibcode:1963Sci...139..835G. doi:10.1126/science.139.3557.835.