 | |
| Names | |
|---|---|
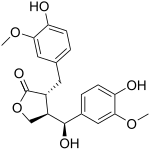
| IUPAC name (7′S,8β,8′α)-4,4′,7′-Trihydroxy-3,3′-dimethoxylignano-9,9′-lactone | |
| Systematic IUPAC name (3R,4R)-4--3-oxolan-2-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H22O7 |
| Molar mass | 374.389 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Hydroxymatairesinol (HMR) is a lignan found in Norway spruce (Picea abies). It is an enterolactone precursor with anticancer activities. In rats, HMR decreased the volume of induced tumours and stabilised established tumours, as well as preventing the development of new tumours. It has also shown anti-oxidant properties in vitro.
HMR's chemical structure is similar to matairesinol. At high concentrations, HMR has estrogenic properties, which are considerably weaker than those of estradiol.
References
- ^ Saarinen, NM; Warri, A; Makela, SI; et al. (2000). "Hydroxymatairesinol, a novel enterolactone precursor with antitumor properties from coniferous tree (Picea abies)". Nutrition and Cancer. 36 (2): 207–16. doi:10.1207/S15327914NC3602_10. PMID 10890032. S2CID 22124446.
- ^ Cosentino, M; Marino, F; Ferrari, M; et al. (August 2007). "Estrogenic activity of 7-hydroxymatairesinol potassium acetate (HMR/lignan) from Norway spruce (Picea abies) knots and of its active metabolite enterolactone in MCF-7 cells". Pharmacological Research. 56 (2): 140–7. doi:10.1016/j.phrs.2007.05.001. PMID 17572100.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |