| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Himeic acid A" – news · newspapers · books · scholar · JSTOR (December 2021) (Learn how and when to remove this message) |
 | |
| Names | |
|---|---|
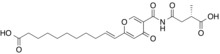
| IUPAC name (E)-11-carbamoyl]-4-oxopyran-2-yl]undec-10-enoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H29NO8 |
| Molar mass | 435.473 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Himeic acid A is a substance with chemical formula C22H29NO8.
References
- Tsukamoto, Sachiko; Hirota, Hiroshi; Imachi, Misako; Fujimuro, Masahiro; Onuki, Hiroyuki; Ohta, Tomihisa; Yokosawa, Hideyoshi (January 2005). "Himeic acid A: a new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp". Bioorganic & Medicinal Chemistry Letters. 15 (1): 191–194. doi:10.1016/j.bmcl.2004.10.012. PMID 15582438.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |