| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Ethyl nitrite | |||
| Other names
1-Nitrosooxyethane Ethyl alcohol nitrite Nitrous acid Nitrous ether Ethyl ester Nitrethyl | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.385 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
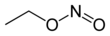
SMILES
| |||
| Properties | |||
| Chemical formula | C2H5NO2 | ||
| Molar mass | 75.067 g·mol | ||
| Boiling point | 17 °C (63 °F; 290 K) | ||
| Solubility in water | 5.07 g/100 ml | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
The chemical compound ethyl nitrite is an alkyl nitrite with a chemical formula C2H5NO2. It may be prepared from ethanol.
Uses
It is used as a reagent with butanone to yield the dimethylglyoxime end product.
Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as Witdulsies, which is sold in pharmacies. It is known as a traditional Afrikaans remedy; the same remedy is apparently made by the Amish in the US. However, FDA has blocked over-the-counter sales of this same remedy, known in the US as sweet nitrite or sweet spirit of nitre, since 1980. Its use has been associated with fatal methemoglobinemia.
Methemoglobinemia is the primary toxic effect of ethyl nitrite. Due to ethyl nitrite's high volatility and faint smell, in the presence of ethyl nitrite vapors, it is easy to breath a high dose of it without realizing, resulting in methemoglobinemia, which may or may not be severe, or even fatal.
References
- "NFPA 704 Ratings for Common Chemicals".
- Semon, W. L.; Damerell, V. R. (1943). "Dimethylglyoxime". Organic Syntheses; Collected Volumes, vol. 2, p. 204.
- "Rulemaking History for OTC Sweet Spirits of Nitre Drug Products". fda.gov. Retrieved 2016-12-26.
- "ETHYL NITRITE - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Retrieved 2017-11-18. "Archived copy". Archived from the original on 2017-12-01. Retrieved 2017-11-18.
{{cite web}}: CS1 maint: archived copy as title (link) CS1 maint: bot: original URL status unknown (link) - "Ethyl nitrite". Haz-Map. Retrieved 2020-08-08.
- Titov, V Yu; Petrenko, Yu M (2005). "Proposed mechanism of nitrite-induced methemoglobinemia". Biochemistry (Moscow). 70 (4): 473–83. doi:10.1007/s10541-005-0139-7. PMID 15892615. S2CID 22906218.