This is an old revision of this page, as edited by Beetstra (talk | contribs) at 15:04, 7 August 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:04, 7 August 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name Chloroethane | |||
| Other names
Ethyl chloride Monochloroethane Chlorene Muriatic ether EtCl UN 1037 | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.755 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C2H5Cl | ||
| Molar mass | 64.51 g/mol | ||
| Appearance | colourless gas | ||
| Density | 0.92 g/cm, liquid | ||
| Melting point | −139 °C (134 K) | ||
| Boiling point | 12.3 °C (285.4 K) | ||
| Solubility in water | 0.6 g/100 ml (?°C) | ||
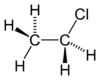
| Structure | |||
| Dipole moment | 2.06 D | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Flammable | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −50°C (closed cup) | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chloroethane or monochloroethane, commonly known by its old name ethyl chloride, is a chemical compound with chemical formula C
2H
5Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.
Production
Ethyl chloride is produced by hydrochlorination of ethylene:
- C2H4 + HCl → C2H5Cl
At various times in the past, ethyl chloride has also been produced from ethanol and hydrochloric acid, or from ethane and chlorine, but these routes are no longer economical. Some ethyl chloride is generated as a byproduct of polyvinyl chloride production. Should demand for ethyl chloride continue to fall to the point where making it for its own sake is not economical, this may become the leading source of the chemical.
Uses
Beginning in 1922 and continuing through most of the 20th century, the major use of ethyl chloride was to produce tetraethyllead (TEL), an anti-knock additive for gasoline. TEL has been or is being phased out in most of the industrialized world, and the demand for ethyl chloride has fallen sharply. It also reacts with aluminium metal to give ethylaluminium sesquichloride, a precursor to polymers and other useful organoaluminium compounds.
Like other chlorinated hydrocarbons, ethyl chloride has been used as a refrigerant, an aerosol spray propellant, an anesthetic, and a blowing agent for foam packaging. For a time it was used as a promoter chemical in the aluminium chloride catalyzed process to produce ethylbenzene, the precursor for styrene monomer. At present though, it is not widely used in any of these roles.
The only remaining industrially important use of ethyl chloride is in treating cellulose to make ethylcellulose, a thickening agent and binder in paints, cosmetics, and similar products.
Ethyl chloride is supplied as a liquid in a spray bottle propelled by its own vapor pressure. It acts as a mild topical anesthetic by its chilling effect when sprayed on skin, such as when removing splinters in a clinical setting. The heat absorbed by the boiling liquid on tissues produces a deep and rapid chill, but since the boiling point is well above the freezing point of water, it presents no danger of frostbite. The vapor is flammable and narcotic, which requires care.
Ethyl chloride is a recreational inhalant drug, sometimes referred to as "Duster". Similar to poppers, ethyl chloride is used as an inhalant (huffed) during sexual activity. In Brazil, it is a traditional (though illegal) drug taken during Carnaval parades, known as "lança-perfume".
Safety
Ethyl chloride is the least toxic of the chloroethanes. Like other chlorinated hydrocarbons, it is a central nervous system depressant, albeit a less potent one than many similar compounds. People breathing its vapors at less than 1% concentration in air usually experience no symptoms. At higher concentrations, victims usually exhibit symptoms similar to those of alcohol intoxication. Breathing its vapors at 15% or higher is often fatal.
Studies on the effects of chronic ethyl chloride exposure in animals have given inconsistent results, and there exist no data for its long-term effects on humans. Some studies have reported that prolonged exposure can produce liver or kidney damage, or uterine cancer in mice, but these data have been difficult to reproduce.
Chloroethane is not classifiable as to its carcinogenicity to humans (Group 3).
Recent information suggests carcinogenic potential; it has been designated as ACGIH category A3, Confirmed Animal Carcinogen with Unknown Relevance to Humans. As a result, the State of California has incorporated it into Proposition 65 as a known carcinogen. Nonetheless, it is still used in medicine as a local anesthetic.
References
- Krause, M.J., Orlandi, F., Saurage, A.T., Zietz Jr., J.R. Aluminum Compounds. Ullmann's Encyclopedia of Industrial Chemistry. 2000. doi:10.1002/14356007.a01_543
- IARC
- IARC
External links
- International Chemical Safety Card 0132
- NIOSH Pocket Guide to Chemical Hazards. "#0267". National Institute for Occupational Safety and Health (NIOSH).
- Template:Ecb
- IARC Monograph "Chloroethane."
- Ethyl chloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)
- National Pollutant Inventory - Chloroethane